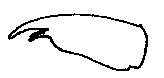
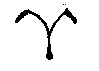Stoneflies - Plecoptera: Perlodidae of Gunnison County, ColoradoArcynopteryx compacta - Arctic Springfly(McLachlan) 1872Updated 12 Jan 21
TSN 103093 HabitatPossibly present at high elevations, 12,000 feet or higher. Look for it!Locations CollectedNowhere in the county yet, found in other areas of Colorado.Good LinksOn this website:Key to Perlodidae Nymphs Other Websites: Photos, Map, Museum specimens, DNA - Barcodinglife.org ReferencesAmore,V; Gaetani,B; Puig,MA and Fochetti,R 2011 New data on the presence of hemocyanin in Plecoptera: Recomposing a puzzle. Journal of Insect Science, 11. HTMLGehrken,U 1989 Diapause termination in eggs of the stonefly Arcynopteryx compacta(McLachland) in relation to dehydration and cold hardiness. Journal of insect physiology, 35(5), 377-385. Abstract: "In the alpine regions of Southern Norway, eggs of the stonefly Arcynopteryx compacta are oviposited in small streams and lake outlets. During ice enclosure, eggs avoid freezing by supercooling and lose about two-thirds of their water content by shrinkage due to osmotic outflux of body water. The eggs enter diapause in autumn at an early stage of embryogenesis. The termination of diapause in 50-55% of eggs kept at 3°C for 3 or 6 months, arises from the effect of chilling on diapause development. The dehydration, however, serves as a diapause-terminating cue. For water contents down to 41%, the termination of diapause seems directly correlated with the degree of dehydration. Treatments with juvenile hormone were also effective in terminating diapause. The termination may therefore be linked to a temporary elevation in juvenile hormone titre by dehydration. Fully hydrated eggs supercool to about -26.5°C, and withstand ice enclosure for 3 days at -22°C. The elevation of supercooling point, water content and depletion of thermal hysteresis-producing proteins correspond with the initiation of postdiapause development. Also ice-enclosure for 3 days at -15 and -18°C proved fatal at this embryonic stage. The loss of cold hardiness could not be related to termination of diapause per se. Eggs remained cold hardy at 3°C as long as the postdiapause development was prevented during the thermally controlled quiescence. The physiological and biochemical mechanisms which underlie adaptation to cold, however, are specific for an early stage of embryogenesis. " Gehrken,U and Somme,L 1987 Increased cold hardiness in eggs of Arcynopteryx compacta (Plecoptera) by dehydration. Journal of Insect Physiology 33(12) 987-991. Abstract: " Eggs of the stonefly, Arcynopteryx compacta, that overwinter in the alpine region of Norwegian mountains, increase their cold-hardiness by dehydration. Eggs enclosed in ice at -22°C survive the loss of about two-thirds of their total water content by shrinkage due to passive diffusion of body water along the concentration gradient. Fully hydrated eggs are killed by freezing at their supercooling point of -26°C, and by direct cooling to -30°C. Dehydrated eggs have a mean supercooling point of -31°C, and survive exposure at -27 and -29°C in ice. Judged from their melting points the eggs do not accumulate low-molecular-weight cryoprotective substances. The difference between freezing and melting points corresponds to a thermal hysteresis of up to 1.8°C. The presence of thermal hysteresis antifreezes may stabilize their supercooled state when enclosed by ice during overwintering. The eggs enter diapause in the autumn, and diapause completion is enhanced both by temperature and time during enclosure in ice. " Hassage,RL 1989 Life histories, behavior and space partitioning in selected species of western North American Plecoptera. pHd Dissertation, University of North Texas. 105pgs. PDF Abstract: "Five species of stoneflies (Zapada haysi, Plumiperla diversa, Taenionema pacificum, Isoperla petersoni, Arcynopteryx compacta) from the North Slope and Interior of Alaska were examined for seasonal patterns of emergence of adults and growth of nymphs. Generally growth was retarded during the winter in this region, and all species except I. petersoni completed growth prior to January. The life cycles of six stonefly species (Prostoia besametsa, Triznaka signata, Sweltsa coloradensis, Isoperla fulva, Skwala parallela, Claassenia sabulosa) are described from northern New Mexico. In this region growth was generally less retarded during the winter than in Alaska; P. besametsa completed all nymphal growth during late fall and winter. Drumming behavior of a Colorado population of Pteronarcella badia was described using an evolutionary framework to explain the maintenance of signal variation in this species. Laboratory experiments were used to explore the effect of intraspecific and interspecific interactions on spatial partitioning in P. badia and Claassenia sabulosa. P. badia exhibited clumping and distributed itself as the surface area of substrate in low densities; however, in the presence of C. sabulosa its distribution was random and different from available surface area. A field study was used to examine spatial partitioning by three New Mexico stonefly species (I. fulva, P. besametsa, T. signata) and to ascertain patterns of microdistribution relating to several abiotic and biotic factors. Generally, there was an interaction of the measured abiotic parameters (current, water temperature, time) with nymphal size. Additionally, void space and sample volume were successfully used to compare biotic densities among leaf and mineral substrates, which were higher in leaf packs than in mineral substrates." Klapálek, Frantisek 1912 Plécoptères. I. Fam. Perlodidae; [monographische Revision. II. Fam. Perlidae; Subfam. Perlinae, Subfam. Neoperlinae; mongraphische Revision] Series Sélys-Longchamps, Edmond de, baron, 1813-1900. Collections zoologiques; catalogue systematique et descriptif, fasc. 4, pt. 1-2. According to Teslenko (2012), quote from page 5: "Klapálek designated Arcynopteryx compacta (McLachlan, 1872) as type species of genus Arcynopteryx. However, he was mistaken and misidentified, when provided (Klapálek 1912, fig. 8, page 13) an illustration of "Arcynopteryx compacta McLachlan", which was actually A. dichroa (McLachlan), thus both taxonomic species actually were involved in the misidentification (International Code of Zoological Nomenclature 1999). The type species of Arcynopteryx fixed (under Article 70.3.2 of the Code) as Arcynopteryx dichroa (McLachlan, 1872), misidentified as Arcynopteryx compacta (McLachlan, 1872) in the original designation by Klapálek (1912). " 


Kondratieff,BC and Baumann,RW 2002 A review of the stoneflies of Colorado with description of a new species of Capnia (Plecoptera: Capniidae). Transactions of American Entomological Society 128 3, 385-401. Abstract:"Eighty-six species of stoneflies are reported from Colorado, including a new species of Capnia. Three new state records are reported, Bolshecapnia milami (Nebeker and Gaufin), Capnura fibula (Claassen), and Isoperla marlynia (Needham and Claassen). Ninety percent of all Colorado stoneflies are typical western North American species, with seven species, Paracapnia angulata Hanson, Taeniopteryx burksi Ricker and Ross, T. parvula Banks, I. marlynia, Acroneuria abnormis (Newman), Perlesta decipiens (Walsh), and Isoperla bilineata (Say) having eastern affinities. Arcynopteryx compacta (McLachlan) is considered circumpolar species." Quote: "Historically this holarctic species was only recorded from Morrison, Jefferson Co.,(Ricker, 1952). Recently a robust population was found: Larimer Co., outlet Chasm Lake, Rocky Mountain National Park, 3,650 m, 16July, 1994, B.C. Kondratieff and R.S. Durfee, 5 males, 4 females (CSU); same but 8 Aug 1997, S. Simonson, 4 males, 4 females (CSU)." Lillehammer,A 1987 Diapause and quiescence in eggs of Systellognatha stonefly species (Plecoptera) occuring in alpine areas of Norway. In Annales de Limnologie-International Journal of Limnology 23(3) 179-184. PDF Abstract: "Eggs of the stoneflies Diura bicaudata and Arcynopteryx compacta, occurring in the middle-alpine vegetation belt, and Dinocras cephalotes not recorded above the sub-alpine, reacted differently to the same temperatures. Those of D. bicaudata and A. compacta had a diapause during the winter. D. cephalotes eggs went into a stage of quiescence when the water temperature was lowered from 16° C to 8° C or below. They remained alive in this stage for about a year, but this quiescence could be broken at anay time when the eggs were transferred to 16 and 20° C. Freezing at -6°° C followed by an increase in the water temperature led to a break in the diapause of A. compacta eggs after five months, but not of D. bicaudata eggs. Eggs of D. bicaudata began to hatch after nine months independently of previous freezing followed by an increase in water temperature. Eggs of D. bicaudata held at a constant temperature of 1.5° C also began to hatch at the same time as those kept at 8° or 16° C. However, the point of 50 % hatching was much delayed at 1.5° C. The egg diapause of A. compacta and D. bicaudata is an adaptation to areas with a severe climate typified by long cold winters and short summers. The quiescence period of D. cephalotes eggs enables the species to survive outside its normal distribution area." Loskutova, O.A. and Zhiltzova, L.A., 2016 Wing and body size polymorphism in populations of the stonefly Arcynopteryx dichroa McL.(Plecoptera: Perlodidae) in the Ural Mountains, Russia. Polar Research, 35(1), p.26596. Abstract: "Specimens from five Arcynopteryx dichroa (McL.) populations were examined to study wing length and body size at different latitudes and altitudes. In northern Europe, female A. dichroa are usually long winged, while males are short winged. During the past 20 years, only two short-winged populations have been found, in a nameless lake, herein called Lake Ozernoe, and in Bolshaya Lagorta Lake, in Russia's Ural Mountains. In the isolated population of Ozernoe Lake (850 m a.s.l.), both sexes were micropterous. In Bolshaya Lagorta Lake (380 m a.s.l.), females were brachypterous. However, at a higher altitude (560 and 760 m a.s.l.), a population was found with macropterous females. Specimens of both short-winged populations had smaller body length than long-winged populations. Our findings give limited support to the idea that stonefly wings are reduced with altitude and latitude and more support to the supposition that small wing size is associated with population isolation resulting from lengthy geological isolation." McLachlan,R 1872 Notes sur quelques espèces de Phryganides et sur une Chrysopa. Bulletin de la Société Impériale des Naturalistes de Moscou. Described as Dictyopteryx compacta. Shepard, WD. and Stewart KW 1983 Comparative study of nymphal gills in North American stonefly genera and a new, proposed paradigm of Plecoptera gill evolution. Miscellaneous Publications of the Entomological Society of America 13:1-57 Illustration of nymphal osmobranchiae (gills) on page 52. Stark,BP and Szczytko,SW 1988. Egg morphology and phylogeny in Arcynopterygini (Plecoptera: Perlodidae) Journal of the Kansas Entomological Society 61(2) 143-160.First Page Abstract: Comparative data are provided for eggs of nine of the eleven recognized genera in the Holarctic tribe, Arcynopterygini, and these data are used to generate a preliminary phylogeny for the group. Four generic clusters (Megarcys/Sopkalia; Frisonia/Perlinodes/Oroperla; Arcynopteryx/Neofilchneria/Skwala and Setvena/Pseudomegarcys) are established primarily from egg data, but resolution of the Frisonia and Arcynopteryx trichotomies required data from other character suites. Detailed illustrations of the epiproct complex are given for six Nearctic genera to provide additional support for the current generic classification of the group and a standardized terminology is proposed for systellognathan Plecoptera eggs. Stewart,KW; Hassage,RL; Holder,SJ and Oswood,MW 1990 Life cycles of six stonefly species (Plecoptera) in subarctic and arctic Alaska streams. Annals of the Entomological Society of America 83(2)207-214. Abstract: Nymphal growth and emergence of adults are described for six species of stoneflies (Plecoptera) found in subarctic and arctic Alaska. The two Nemouridae studied are semivoltine; adults of Zapada haysi (Ricker) are present from May to July and adults of Nemoura arctica Esben-Petersen occur from June to July. The remaining four species are univoltine. Plumiperla diversa (Frison) (Chloroperlidae) has most of its growth occurring during the summer with emergence the following May-September. Taenionema pacificum (Banks) (Taeniopterygidae) completes nymphal growth by the end of January and has an early emergence (April-June). Adults of Arcynopteryx compacta (McLachlan) (Perlodidae) are present from May to August, and growth of nymphs is rapid during summer and fall. Isoperla petersoni Needham ∓ Christenson (Perlodidae) adults are present from June to mid-August, and nymphal growth is interrupted by winter and resumes in the spring; the three other univoltine species studied tend to complete growth before the onset of winter. Growth of these six species is tied to seasonal temperature variation. Stewart,KW and Stark,BP 2002 Nymphs of North American Stonefly Genera. 2nd edition The Caddis Press, Columbus, Ohio. 510 pages. Illustration of nymph on page 365 figure 14.1. Teslenko,VA 2012 A taxonomic revision of the genus Arcynopteryx Klapálek, 1904 (Plecoptera, Perlodidae). Zootaxa, 3329, 1-18. PDF At the end of this paper the author redescribes Arcynopteryx compacta as Skwala compacta. Abstract: " Four known Arcynopteryx species are redescribed from the types and newly acquired material. Illustrations of the male and female genitalia, head and pronotal patterns and eggs are used to support species descriptions. Dictyopteryx compacta (McLachlan, 1872) is transferred to Skwala Ricker, 1943 with the valid name Skwala compacta (McLachlan, 1872) comb. nov., and S. pusilla (Klapálek, 1912) is placed as a junior synonym of that species. For genus Arcynopteryx type species is fixed (under Article 70.3 of the Code) as Arcynopteryx dichroa (McLachlan, 1872), misidentified as Arcynopteryx compacta (McLachlan, 1872) in the original designation by Klapálek (1912). " Theissinger,K; Feldheim,KA; Seitz,A and Pauls,SU 2009 Isolation and characterization of 11 polymorphic trinucleotide microsatellite markers in the stonefly Arcynopteryx compacta (Plecoptera: Perlodidae) Molecular Ecology Resources 9(1)357-359. Abstract: We describe the isolation of eleven polymorphic trinucleotide microsatellite loci from the stonefly Arcynopteryx compacta. Loci were highly variable with 3 to 14 alleles (mean = 6.45). Observed heterozygosity ranged from 0 to 0.867. Seven loci showed significant deviation from Hardy-Weinberg equilibrium across both populations. There was no evidence for null alleles, and thus, Hardy-Weinberg departures could have resulted from genetic structure between populations or subpopulations. No linkage between loci was found. The eleven loci should prove highly informative for population genetic studies. Illustrations
|